Giannis
First Class Player
- Joined
- Jun 25, 2019
- Runs
- 2,665
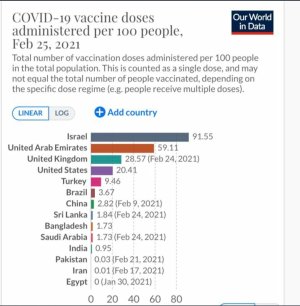
There's so many vaccines that are being developed and some have already been approved, was wondering what y'all might prefer (if required).
Pfizer/BioNTech vaccine - 90 - 95% effective, 2 shots and recently approved. Developed by a Turkish couple.
Moderna - 95% effective, 2 shots and will be approved next week. Developed by an African-American woman.
Oxford/Astrazeneca - 50-70% effective, 1 shot, controversial. FDA and other developed countries may not approve it yet as they've been accused of poor research and cutting corners however the UK really wants to push for national pride reasons.
Johnson&Johnson - 1 shot, might get approved in February-March. It's not MRNA based unlike the first two, I might get this a year from now
GSK/Sanofi - Another European attempt at the vaccine, push until end of 2021 due to a lab mistake
Sinopharm - Chinese vaccine, I think most of in the west may not get this but perhaps people in Asia will.
Sputnik V - Russian vaccine and first to be registered although it's quite controversial and now the British are trying to combine it with their Oxford vaccine. I don't see this getting approved here in America but I think Pakistanis might get access.
There's also an Australia attempt at making a vaccine but resulted in an a positve HIV test.
Pfizer/BioNTech vaccine - 90 - 95% effective, 2 shots and recently approved. Developed by a Turkish couple.
Moderna - 95% effective, 2 shots and will be approved next week. Developed by an African-American woman.
Oxford/Astrazeneca - 50-70% effective, 1 shot, controversial. FDA and other developed countries may not approve it yet as they've been accused of poor research and cutting corners however the UK really wants to push for national pride reasons.
Johnson&Johnson - 1 shot, might get approved in February-March. It's not MRNA based unlike the first two, I might get this a year from now
GSK/Sanofi - Another European attempt at the vaccine, push until end of 2021 due to a lab mistake
Sinopharm - Chinese vaccine, I think most of in the west may not get this but perhaps people in Asia will.
Sputnik V - Russian vaccine and first to be registered although it's quite controversial and now the British are trying to combine it with their Oxford vaccine. I don't see this getting approved here in America but I think Pakistanis might get access.
There's also an Australia attempt at making a vaccine but resulted in an a positve HIV test.
Last edited by a moderator:







 get outta here. The American and Russian vaccines are ~95% effective. Even the FDA won't approve the British vaccine lol.
get outta here. The American and Russian vaccines are ~95% effective. Even the FDA won't approve the British vaccine lol.